It is an organometallic reaction which leads to the formation of alkanes by coupling of two alkyl halides in presence of sodium metal. The reaction was discovered by Charles- Adolphe Wurtz in 1855.
![]()
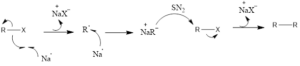
Mechanism of action: It is a reaction which involves free radical formation. Firstly there is single electron transfer, sodium metal transfers electron to alkyl halide. Then alkyl halide forms radical along with sodium halide salt. Another sodium transfers its electron and there is the formation of carbanion of an alkyl radical. Then another alkyl halide is attacked upon by alkyl carbanion and this involves nucleophilic substitution reaction (SN2). Finally, the higher alkane is formed by coupling along with formation of another molecule of sodium halide salt.
Wurtz- Fittig reaction is the extension of Wurtz reaction which was discovered by Rudolph Fittig in the 1860s. This reaction forms substituted aromatic compound from aryl halide and an alkyl halide in presence of sodium.
![]()
There are certain limitations to Wurtz reaction, it does not synthesize asymmetric alkanes if different alkyl halides are taken, then a mixture of alkanes are formed which are difficult to get separated. The reaction forms side product alkene which decreases the yield of alkane and reaction can’t be used to form methane whereas Wurtz -Fittig reaction can form asymmetric products.