Online Retrosynthesis Homework Help
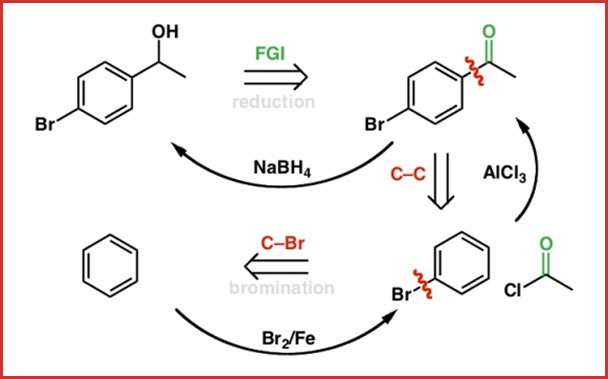
Retrosynthesis Assignment Help
Retrosynthesis is designing a reverse synthesis of the organic compound. This helps us to find the way of synthesis for that compound. Retrosynthesis give us an idea about the synthetic steps of a complex compound as well. Thus by Retrosynthesis, we can convert the target molecule into its simple precursors.
We are here to help you in designing Retrosynthesis of any organic molecule of your interest. Feel free to contact us and share your situation and goal related to retrosynthesis homework and assignment help problems.
Upload Homework/Assignment

Online Organic Chemistry Tutor
Retrosynthesis Analysis Process
Identify the Target Molecule (TM)
Identifying the Target molecule is the first step in retrosynthetic analysis. Understanding its functional groups, stereochemistry, and overall complexity is pivotal for planning the synthesis.
Disconnection Approach
The disconnection approach is the core of retrosynthetic analysis, where bonds in the target molecule are disconnected to generate simpler fragments or synthons.
There are two main types of Disconnections:-
- Functional Group Disconnections
- Carbon-carbon Bond Disconnections
Select Key Disconnections
While considering the practicality of the resulting intermediates, prioritize key disconnections that simplify the structure most effectively. The selected disconnection should be ideal for leading stable, recognizable intermediates that can be traced back to available starting materials.
Identify Reagents and Reactions
Determine the corresponding synthetic equivalent. Choose well-known, reliable, and compatible reactions with the functional groups present in the molecule.
Repeat the Process
The retrosynthetic analysis for each synthon will continue until all fragments are reduced to simple, commercially available starting materials. This iterative process often requires multiple rounds of analysis, particularly for complex target molecules.
Consider Alternative Routes
Retrosynthetic analysis often involves exploring numerous pathways to identify the most efficient and practical synthesis route. To optimize the synthesis, consider alternative disconnections, reagents, and strategies.
Forward Synthesis Planning
convert the retrosynthetic plan into a forward synthesis pathway once the retrosynthesis analysis is complete. This step may involve planning the actual sequence of chemical reactions needed to synthesize the target molecule from the identified starting materials.
Rely on our top-notch Retrosynthesis help services to master complex concepts with ease. Our specialized retrosynthesis assignment help is designed to guide you through the complexities of organic chemistry. With expert tutors, you’ll learn to break down complex molecules, design synthetic pathways, and excel in your coursework. Get the retrosynthesis to help support you in mastering retrosynthesis and boosting your academic performance.
FAQs Related to Retrosynthesis:
Retrosynthesis is a method used in organic chemistry to break down complex molecules into simpler starting materials, working backward from the target compound to discover a synthetic pathway. It’s essential for designing efficient and logical synthetic routes in organic chemistry.
Yes! We cover various retrosynthesis strategies, including:
- Functional group interconversions
- Protecting group strategies
- C-C bond formation
- Named reactions (e.g., Diels-Alder, Grignard, Aldol reactions) We tailor our approach based on your level of knowledge and the specific requirements of your assignment.
Absolutely! We specialize in helping students break down complex multi-step synthesis problems into manageable parts. We can help you plan the synthesis from scratch, or provide feedback and advice on your proposed solutions.
Basic knowledge of organic chemistry is important, especially understanding reactions, functional groups, and mechanisms. However, if you’re new to retrosynthesis, we can provide foundational explanations to get you up to speed before diving into more complex problems.
Yes, we can assist with retrosynthesis problems for advanced courses and research projects. Whether you’re working on a complex synthetic pathway for a research paper or a lab project, our tutors can provide expert guidance.
Yes, we offer tutoring specifically geared towards preparing for exams. We’ll focus on common retrosynthesis question types, teach you how to approach these problems efficiently, and provide mock questions for practice.
