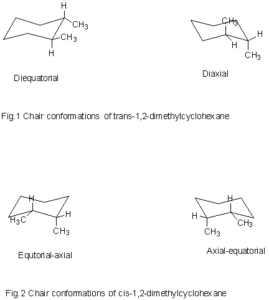
In the given figure various possible chair conformations of 1,2-dimethylcyclohexane are drawn. There are two possibilities that are cis or trans, but the position of the methyl group on axial or equatorial bond on cyclohexane determines whether the compound is cis or trans. Like in given figure no. 1, if both methyl groups are attached to an equatorial (diequitorial) or axial (diaxial) position then it would be called trans-1,2-dimethylcyclohexane because one bond is up and another bond is down, however, both are axial or equatorial. In figure no. 2, one methyl group is on equatorial and other is on axial position but both are on the downside, or both may be on the upside so this case would be considered as cis, and the name would be cis-1,2-dimethylcyclohexane.