A tetrahedral carbon with four different substituents shows the enantiomerism with different three dimensional isomeric structures. The carbon bearing four different substituents is called as chiral or asymmetric carbon.
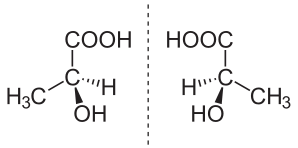
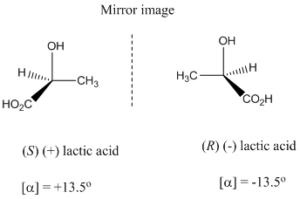
A molecule with one asymmetric carbon shows two enantiomers which are mirror image of each other and non-superimposable.
Enantiomers are optically active and they rotate the plane polarized light either in left or right direction. So enantiomers have identical physical properties except the direction of optical activity. Generally enantiomers have equal but opposite optical rotatory power. The enantiomer rotate plane polarized light to right direction called dextrorotatory and optical activity denoted as “+” whereas its mirror image rotate plane polarized light to left direction called levorotatory and optical activity denoted as “-”.
The atoms in enantiomers are oriented in the space in a definite arrangement which is referred as configuration and it can be represented by “R” and “S” absolute configuration. Cahn–Ingold–Prelog system, called the sequence rules, is used to specify the absolute configuration at the stereogenic center.
Stereoisomers with two or more chiral carbons that are neither mirror image nor superimposable are called diastereomers which differ not only in optical activity but also have different physical properties.