The Perkin reaction is a condensation reaction of organic chemistry. It involves the formation of α, β unsaturated carboxylic acid from aromatic aldehydes and anhydride in presence of a base catalyst which is alkali salt of the acid.
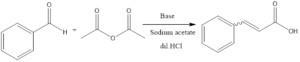
The reaction was first discovered by William Henry Perkin in the 19th century, who was an English Chemist. The reaction involves aldol condensation, in this reaction enolate ion of aldehydes or ketone leads to the formation of β hydroxy aldehydes or ketone, and further, it involves dehydration to form conjugated enone.
In the reaction procedure, it involves heating of aldehyde along with acid anhydride at temperature 180°C. The reaction then results into formation of anhydride. The unsaturated acid is formed by hydrolysis of anhydride in presence of dilute HCl.
Mechanism of action: it involves many additions as well as eliminations. Firstly, there is the removal of the proton by carboxylate ion to form carbanion. Then there is the formation of tetrahedral intermediate by nucleophilic addition of formed carbanion to the carbonyl group of aldehydes. The acetic acid generated then protonates tetrahedral intermediate. Then the water molecule is removed from the hydroxy derivative. Finally, hydrolysis takes place which forms unsaturated acid.
Perkin reaction is used for the formation of cinnamic acids which are naturally present in cinnamon and shea butter. It is used for the formation of phytoestrogenic stilbene resveratrol in the laboratory.