Elimination Vs. Substitution
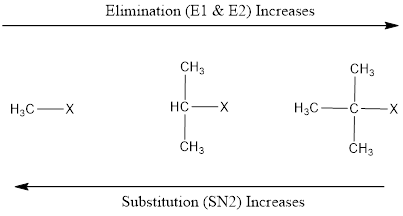
Substrate Structure (Primary, secondary or tertiary) determines whether elimination or substitution will take place. 2. Less polar solvent (like ethanol) and higher temperature favours elimination. 3. Concentrated KOH and NaOH favours elimination. (Strong base favours elimination and good nucleophile favours substitution).
Factors affecting acidity of carboxylic acids
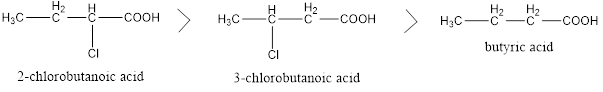
Two types of groups can be there in structure of carboxylic acid, one is electron withdrawing and other is electron donating. Electron donating groups decreases the acidity and Electron withdrawing groups increases the acidity of carboxylic acid, and also their nearness to carboxylic group creates more impact on acidity compared to the groups away from […]
Factors affecting basicity of Primary Amines
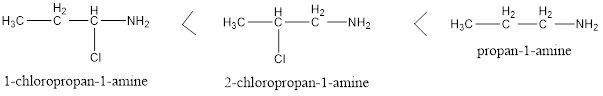
Two types of groups can be there in the structure of primary amines, one is electron withdrawing and other is electron donating. Electron donating groups increases the basicity and Electron withdrawing groups decreases the basicity of amines as their basicity depends on the availability of free lone pair present on nitrogen, electron donating group increases […]
Bartoli Indole Synthesis
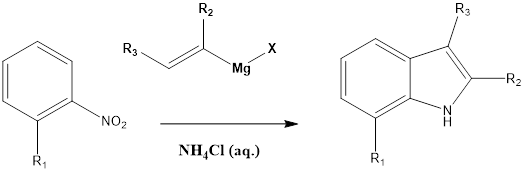
This reaction involves the formation of substituted indoles from nitroarene in presence of an excess of vinyl Grignard reagent and aqueous ammonium chloride. The reaction was discovered by Giuseppe Bartoli and his team in the year 1989. The ortho-substituted nitroarene is preferred as they give a good yield. The bulkier group also affects yield, as […]
Corey-Kim Oxidation
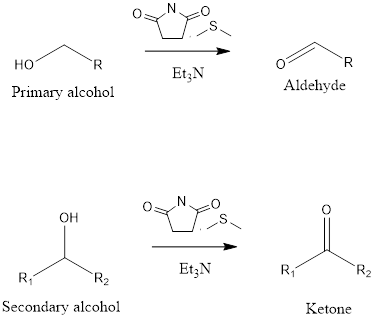
It is an organic reaction to form aldehydes and ketones from primary and secondary alcohol. The reaction takes place in presence of N-chlorosuccinimide(NCS), dimethylsulfide(DMS) and triethylamine(TEA). The oxidation reaction was discovered by Nobel laureate Eliar James Corey, American chemist and Choung Un Kim, Korean American chemist in the year 1972. In the mechanism of this […]
Ullmann Reaction
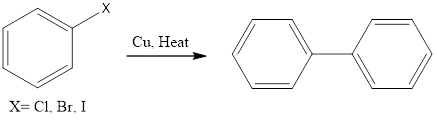
Ullmann reaction is coupling organic chemistry reaction which forms biaryl by coupling of two molecules of aryl halide in the presence of copper metal and elevated temperature (200°C). The reaction was discovered by Fritz Ullmann. Mechanism of this organic reaction is not clear exactly. There are two proposed mechanisms first is the radical mechanism which […]
Jones Oxidation
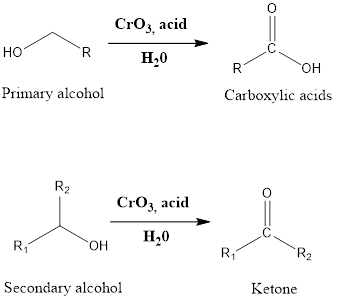
It is an oxidation reaction of organic chemistry which leads to the formation of carboxylic acid and ketone. The primary alcohol in presence of chromic trioxide, and sulfuric acid in a mixture of acetone-water (which is also referred as Jones reagent) forms carboxylic acid while secondary alcohol in presence of Jones reagent leads to the […]
Johnson-Claisen Rearrangement
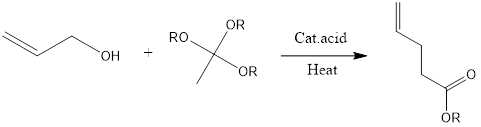
Johnson-Claisen rearrangement is organic chemistry reaction to form unsaturated ester from allylic alcohol and trialkyl orthoacetate in presence of mildly acidic conditions and heat. The reaction was discovered in 1970 by W.S Johnson and its co-workers. Mechanism of reaction– Firstly alkoxide group is protonated of orthoacetate. Then molecule of alcohol is released from protonated alkoxide. […]
Biginelli Reaction
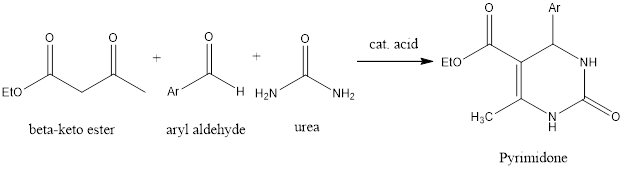
Bignelli reaction is ring forming organic chemistry reaction which forms pyrimidones from three components aldehyde, β-keto ester, and urea in the presence of acidic conditions. The reaction was discovered by Italian chemist Pietro Biginelli in 1891. Mechanism of reaction – Firstly, in reaction condensation of aldehyde and urea takes place, as it happens in […]
Eschenmoser-Claisen Rearrangement

Eschenmoser-Claisen rearrangement is the organic chemistry reaction which leads to the formation of α, β unsaturated amide from allylic alcohol in presence of heated N,N dimethylacetamide dimethyl acetal. The reaction is named after Albert Eschenmoser who discovered this reaction in 1964. This reaction is also used in synthesis of morphine (opioid analgesic). Mechanism of organic […]